compressed air microbial testing|compressed air sampling methods : dealer Testing your compressed air for microbial contamination requires the appropiate sampling methods. Use ISO 8573-7 validated equipment. If you ever like to test your luck with online slots for real money - Bovada.lv is one of the eldest and most trusted operator websites, providing gambling services since 2011. Here you can play any of the slots above for real cash, with bets starting from $0.01 per slot line and ending with high limits - $100 per spin or even higher.
{plog:ftitle_list}
WEBBig Bucks Bandits Megaways. Saddle up and head to the Wild West in the rootin’-tootin’ Big Bucks Bandits Megaways™. Look out for the Jackpot Respins Bank Heist feature, .
Choosing a method for testing microbial contamination in compressed air and gases requires evaluating which results the facility is requesting. Does the facility simply want .
The Safe Quality Food Institute (SQFI) has introduced a new Safe Quality Food Manual (ISO 22000) in the U.S. According to the standard, food processors must test annually for factors including particulate, water, oil, .
micro testing on compressed air
iso 8573 7 free download
The Microbial Impaction Sampler (Pinocchio Super II) is used by manufacturers to test their compressed air systems. The analysis of .Testing your compressed air for microbial contamination requires the appropiate sampling methods. Use ISO 8573-7 validated equipment.The Safe Quality Food Institute (SQFI) has introduced a new Safe Quality Food Manual (ISO 22000) in the U.S. According to the standard, food processors must test annually for factors .Compressed air — Part 7: Test method for viable microbiological contaminant content 1 Scope This part of ISO 8573 specifies a test method for distinguishing viable, colony-forming, .
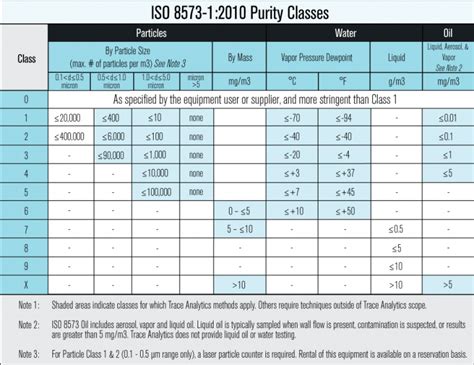
Part 1 describes 3 primary contaminant types with 9 classes with 9 most liberal to 0 the most exacting. Also, compressed air purity is classified as A, B, or C. Class A describes solid particles, B, liquid water/humidity; C oil particles. This harmonization created an effective method to communicate requirements for a compressed air system between the end user, filter and compressor manufacturer, and testing laboratory. Different Methodologies .Compressed air microbial testing or Bioburden Testing per ISO 8573-7 or ISO 14698 is generally conducted by the pharmaceutical, medical device and food industries. Microbial contaminants found in the compressor or compressed .CAMTU Compressed Air Microbial Test Unit. British Compressed Air Society has produced a specification for dewpoint (-40F/C), oil removal <0.01mg/m3 and particulate removal (including microbiological particles) 0.1-0.5 microns. Request white paper by Lee Scott, “Reducing
Testing and monitoring compressed air and other process gases, such as gaseous and liquid nitrogen, oxygen, argon, and carbon dioxide, that come into direct contact with pharmaceutical drugs during the .Parker's Compressed Air Microbial Test Unit (CAMTU) offers many features and benefits including: Lightweight and ergonomically designed for ease of use; Constructed of durable polypropylene - easily sanitized; Pre-filled petri dishes with specialized tryptic soy agar designed to hold up to compressed air flow/pressure;CAMTU Replacement Spray Bottle (1) Compressed Air Microbial Test Unit (1) Potato Dextrose Agar Pre-filled Plates - *Ships 2-Day Air on Ice* (1) Tryptic-Soy Agar Pre-filled Plates - *Ships 2-Day Air on Ice* (1) Unfilled Petri Dishes for CAMTU (1) (+) show 5 more. Mfr. Item No.
Compressed Air microbial Test Unit: UNSPSC: 41000000: Product Details. Used to identify sources of contamination in compressed air. To obtain a sample, simply plug the connection tubing into the sample point on the compressed air system, insert a specially designed petri dish into the CAMTU, close the CAMTU, open the shutoff valve and expose .Compressed air isn’t inherently clean; like the ambient environment it’s drawn from, the air in your compressor system is filled with a variety of particles, aerosols, and vapors that can contaminate end processes and products, as well as harm machinery and other equipment. Employing a Standard for Compressed Air Testing Selecting ISO 8573-1 as the basis for compressed air quality monitoring and testing is the obvious choice, since it provides a common language that all involved parties can use. . Nor does ISO 8573-1 include purity classes for microbiological contaminants. Testing methods are provided in Part 7 .
Testing Compressed Air Systems for Contamination in the Food Manufacturing Environment . offers prompt and reliable microbial testing for compressed air utilizing the Compressed Air Microbial Test Unit (CAMTU). Developed by Parker Hannifin, a corporation specializing in motion and control technologies, CAMTU is widely recognized as the most .in compressed air — Sample test report Once the solid particle content in accordance with ISO 8573-4 has been established, a tabulated test report (see Figure A.1) is used to identify those particles present as viable microbiological CFUs in a sample of air taken from the compressed air system under investigation.Compressed Air Microbial Test Unit (CAMTU) Operation 1. Open sample tap long enough to purge compressed air until visibly free of moisture and particulates. 2. Assemble the tubing to the shutoff valve, regulator and CAMTU as shown in Figure 1 . The objective of this project was to compare the compressed air sampling capability of the newly developed Compressed Air Microbial Testing Unit (CAMTU) to a reference Andersen single stage viable particle sizing sampler for recovery of high pressure aerosolized Micrococcus luteus cells. Materials and Methods Bacterial Cultures
compressed air testing parameters
Microbial air monitoring ensures aseptic environments, vital in food, beverage, and cannabis manufacturing. . When a microbial test method (S.O.P.) needs to be set up for a new product or improved for a product that demonstrates antimicrobial effects and/or filtration issues, our applications laboratory can develop a method that is compliant . assessments is ISO 8573 Compressed air — Part 7: “ Test method for viable microbiological contaminant content ” (7). When sampling compressed air for microorganisms, it is important that the .In active monitoring, a microbial air sampler is used to force air into, or onto its collection medium (e.g., petri dish with nutrient agar-based test media) over a specified period of time. The collected culture can then be incubated and analyzed ( i.e., count bacterial and/or fungalCAMTU Compressed Air Microbial Test Unit. 3 www.balston˜lters.com Unlike the conventional agar plate, this unique CAMTU agar plate offers greater dispersion of the compressed air over the agar as a result of an improved air flow path through .
According to the Compressed Air and Gas Institute (CAGI) and the International Organization for Standardization (ISO), the three major contaminants in compressed air are solid particles, water, and oil. CAGI .We can accommodate testing specifications like ISO 8573, ISO 14698, NFPA 1989, NFPA 99, OSHA, CGA, CSA, ISPE, USP and many more. Contact us to discuss your compressed air testing and environmental monitoring needs.
Once the compressed air microbial monitoring plan is approved, a sampling procedure that provides the company with the results suitable to its limits and specifications needs to be established. . Compressed Air Microbial Testing Unit Detection Kit [Manual]. (07/2015) SAS 180 Air Sampler [Manual]. (03/2007) Macher, J. (1989). Positive-Hole .
Microbial air monitoring in cleanrooms is the process of sampling and analyzing the air to detect and quantify the presence of microorganisms such as bacteria, fungi, and other microbes. This is a crucial requirement in cleanrooms and cleanroom-associated areas in various industries, such as the pharmaceutical industry, medical device .CAMTU Compressed Air Microbial Test Unit. 3 www.balston˜lters.com Unlike the conventional agar plate, this unique CAMTU agar plate offers greater dispersion of the compressed air over the agar as a result of an improved air flow path through . The Parker Balston Compressed Air Microbial Test Unit (CAMTU) is a lightweight, easy to use device capable of sampling compressed air systems for microbes. The unit requires no electricity and has a quick sampling time of 20 seconds. The CAMTU is an ideal device to incorporate into your Good Manufacturing Practices program for monitoring all .ISO 8573 compressed air testing provides limits for particles, water, and oil. . Test from the compressor or point of use. Watch our online sampling video for Manufacturing Air Testing. Microbial and Environmental Testing Available! see more Kits. ISO 8573-1 Purity Classes. ISO 8573-1:2010 COMPRESSED AIR CONTAMINANTS AND PURITY CLASSES;


Meanwhile ISO 8573-7 focuses on Microbial testing of compressed air. While ISO 8573-7 does not require a specific sampling method, it does require Colony Forming Unit [CFU] enumeration. A specific type of Impactor sieve sampler, the SAS Pinocchio Super II which is utilized by Trace Analytics it can provide a quantitative sampling procedure for . These essential PPE items are necessary to avoid cross contamination when testing compressed air microbial samples using aseptic technique. If the technician has a beard or long hair, hairnets are always recommended. Eye protection is important for protecting eyes while using disinfectant sprays and preparing the compressed air for sampling. 5.3 Test for Oil and Moisture Content Note: These tests are applicable for Compressed air points only. 5.3.1 This test shall be performed by the external testing laboratory as per protocol or by using gas detector tubes (Gastec). 5.3.2 Frequency Sample Point from Compressed Air Generation System: Once a Month
CAMTU Compressed Air Microbial Test Unit. British Compressed Air Society has produced a specification for dewpoint (-40F/C), oil removal <0.01mg/m3 and particulate removal (including microbiological particles) 0.1-0.5 microns. Request white paper by Lee Scott, “Reducing
compressed air testing guidelines
compressed air testing food industry
compressed air sampling methods
compressed air sampler for microbiology
Não se preocupe!Confira na íntegra o Carimbó dá Sorte, exibido no domingo, 12 de Março de 2023.🍀 Acesse nos.
compressed air microbial testing|compressed air sampling methods